Online: 3084-8008
Instructions for authors
The instructions for authors adhere to the guidelines set forth by the International Committee of Medical Journal Editors (ICMJE) (https://www.icmje.org/about-icmje/), the World Association of Medical Editors (WAME) (https://www.wame.org/), and the Committee on Publication Ethics (COPE) (https://publicationethics.org/).
Manuscript submission to Medical Education & Clinical Practice
Manuscript sections
The first manuscript submission must include the following documents:
- Cover letter.
- Author Disclosure Form ( download here ).
- Title page.
- Manuscript including tables and figures (in Word format).
- Individual figure files in their original format (if applicable).
- Supplementary material (if applicable).
- Reporting checklist according to the study design (if applicable).
Title page
The title page must include the following information:
- Title: The article must include a title in both Spanish and English. A maximum length of 20 words is recommended.
- Short title: A brief version of the title, not exceeding 7 words, should be provided in the language of the manuscript.
- Authors’ full names: Each author must provide their full name. Authors may indicate their preferred name format for publication; otherwise, the standard format (first name followed by last name) will be applied.
- Affiliations: The institutional affiliation of each author must be specified, including the city and country.
- ORCID iD: All authors are required to provide their ORCID identifier.
- Corresponding author contact information: The corresponding author must provide a valid email address (see Corresponding Author section).
- Prior presentation of the content: If the article was previously presented at a conference, symposium, or academic event, or is part of a thesis, this must be clearly indicated along with relevant details.
- Individual contributions: For manuscripts with multiple authors, the specific contribution of each author to the development of the manuscript must be clearly stated (see Authorship criteria and Contributions section).
- Funding: Authors must state whether the study was self-funded or supported by external funding. If applicable, the full name of the funding organization must be provided (see Funding Sources section).
- Conflict of interest statement: Authors must disclose any personal, institutional, or financial relationships that could potentially influence the objectivity of the research (see Conflict of interest Disclosure section).
- Generative Artificial Intelligence use statement: If artificial intelligence tools were used in the preparation of the manuscript or the conduct of the study, their use must be explicitly described (see Generative Artificial Intelligence (AI) use statement section).
- Data availability statement: Detailed information must be provided regarding the conditions for accessing the data used in the study, including their public availability, any usage restrictions, or the procedure for requesting them (see AI, open access and data section).
Declarations
The following examples are provided as a guide for appropriately incorporating the required statements on the title page:
- Funding
“This study was funded by [Name of Institution] ([Grant Number]); [Name of Institution] ([Grant Number]); and [Name of Institution] ([Grant Number])”. If the study did not receive any funding, the following statement must be included: “This study did not receive any funding.”
- Conflict of interest statement
Authors must disclose any personal, institutional, or financial situation that could influence the objectivity of the study. If the authors have no conflicts of interest, the following statement must be included: “The authors declare no conflicts of interest.”
- Generative Artificial Intelligence use statement
“During the preparation of this work, the author(s) used [Name of AI tool or service] for the purpose of [Specify purpose]. After using this tool, the author(s) reviewed and carefully edited the content as needed and take full responsibility for the content of the publication”. If the authors did not use artificial intelligence, the following statement must be included: “The authors declare that no artificial intelligence was used in any part of the manuscript preparation process”.
- Data availability statement
Authors must provide complete information regarding the accessibility of the data used in the study.
- If the data are stored in a repository, authors must include the direct web link and the corresponding DOI: “The data generated and/or analyzed during the present study are available in the [Name] repository at the following link: [Web link].”
- If access to the data requires contacting the corresponding author: “The data used and/or analyzed during the present study are available from the corresponding author upon reasonable request.”
- When access restrictions apply, authors must clearly explain the reasons justifying the protection of the data: “The data generated and/or analyzed during this study are not publicly available due to [Reason why the data are not public], but they may be requested from the corresponding author.”
- If the data used are contained within the article, authors must include the following: “All data generated or analyzed during this study are included in supplementary material.”
- If the data are publicly accessible, authors must indicate the access method and provide the direct web link: “The data supporting the findings of this study are available from [name of the third party] at the following link: [web link].” If the data belong to an institution and restrictions apply, authors must clearly declare them.
- Use “Not applicable” if data sharing does not apply to this article because no datasets were generated or analyzed during the study.
- Ethical approval and consent to participate
Case reports must indicate that consent for publication was obtained or, alternatively, that approval was granted by an ethics committee or institutional review board, specifying the name of the committee and the corresponding reference number.
Manuscript
The manuscript must include the following components:
- Abstract: An abstract must be provided in both English and Spanish, regardless of the language of the manuscript. It is mandatory for both original and brief original articles. The abstract must meet the following requirements: i) it must not exceed 250 words, and ii) it must be written in a structured format, following this order:
Objectives: State the main objective(s) of the study.
Methods: Summarize the study design, data sources, eligibility criteria, analytical procedures, and the key variables assessed.
Results: Present the most relevant findings of the study, including the main estimates and their measures of uncertainty (confidence intervals or p-values, as appropriate).
Conclusions: Provide a concise interpretation of the findings and their implications.
Protocol registration: The protocol registration number, the registry platform used (e.g., PROSPERO), and the corresponding link (if available) must be provided. This requirement applies only to systematic reviews or articles reporting the results of a health intervention in human participants. - Keywords in English and Spanish: Between three and ten keywords in both English and Spanish must be included, separated by semicolons. Authors are encouraged to use the Medical Subject Headings (MeSH) of the National Library of Medicine (https://www.ncbi.nlm.nih.gov/mesh) for English keywords, and the Descriptores en Ciencias de la Salud (DeCS) of BIREME (https://decs.bvsalud.org/es/) for Spanish keywords.
- Main text: The length and structure of the main text depend on the type of manuscript submitted (see Manuscript Types and Requirements section).
- References: References should be limited to those explicitly cited in the text. The in-text citation callouts must use consecutive superscripted bracketed numbers placed before punctuation marks. When citing two consecutive references, they should be separated by a comma (e.g., [1,2]), and when citing a sequence of more than two references, they should be separated by a hyphen (e.g., [1–3]). The references must appear at the end of the manuscript, numbered consecutively in the order in which they are cited, and formatted in accordance with the Vancouver style, including the Digital Object Identifier (DOI), as recommended by the International Committee of Medical Journal Editors (ICMJE). For references with more than six authors, list the first six followed by et al. The use of reference management software is strongly recommended to ensure accuracy and consistency.
Manuscript types and requirements
The following types of manuscripts can be submitted to Medical Education & Clinical Practice:
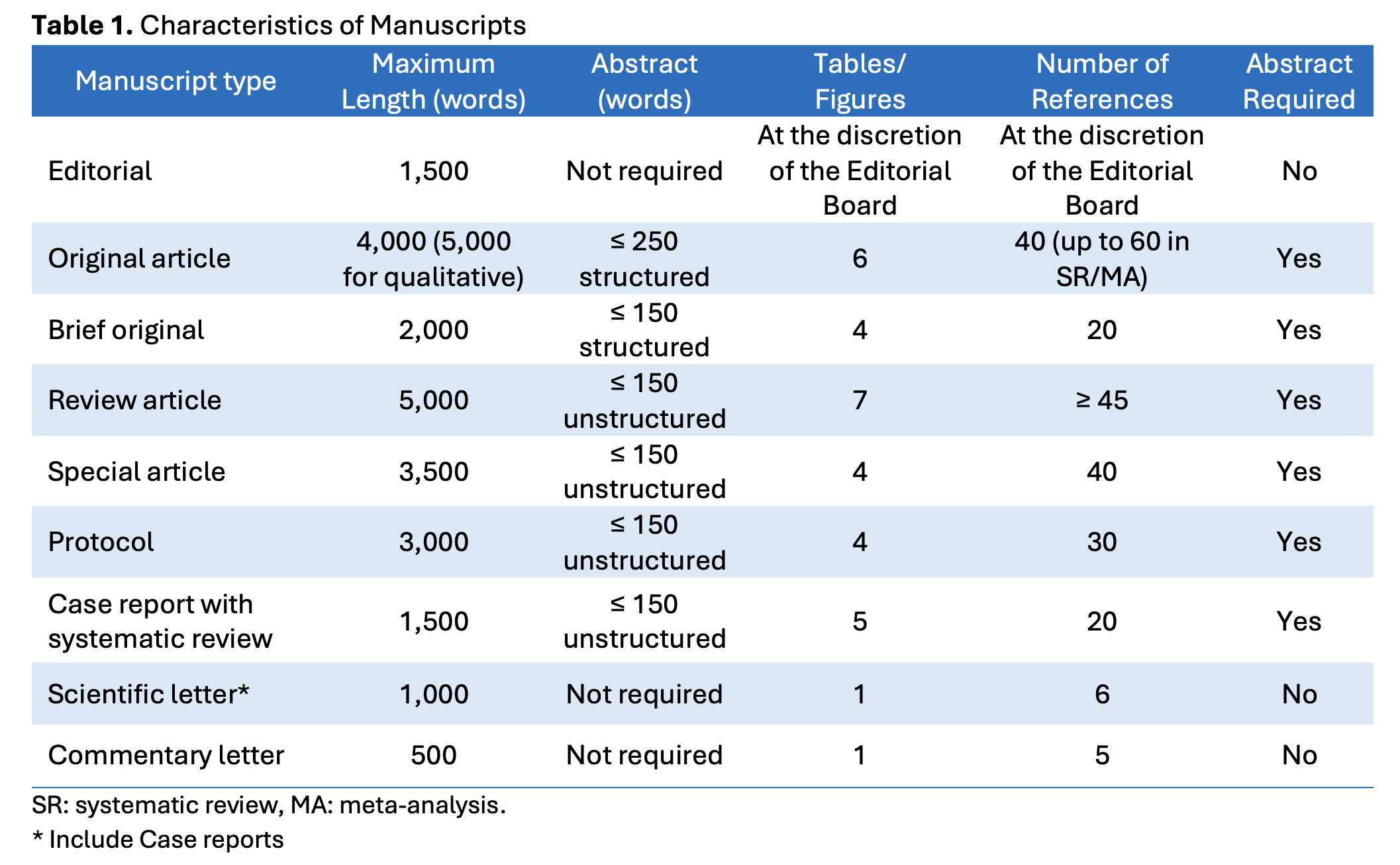
Detailed information about each manuscript type is provided below.
Editorial
Editorials are typically invited by the Editorial Committee and authored by experts in a specific field. Their content should address topics of interest related to medical education, clinical practice, public health, or issues pertaining to the editorial policy and management of the journal. Editorials are reviewed by the Editorial Committee and may be sent for peer review if deemed necessary. The maximum length is 1,500 words, excluding references. Editorials should not include an abstract or keywords.
Original articles
Original articles report the results of scientific research in medical education, epidemiology (including secondary data analyses), and clinical practice. This category includes qualitative studies and systematic reviews with or without meta-analysis conducted using rigorous and clearly defined methodologies. Manuscripts must address topics relevant to the journal’s scope, particularly those related to clinical practice and medical education. The maximum length for original articles is 4,000 words, excluding the abstract, acknowledgments (see Authorship Criteria and Contributions section), figure legends, tables, references, and supplementary material. A structured abstract of no more than 250 words is required. Manuscripts may include up to six tables or figures and a maximum of 40 references. For qualitative studies, the word limit can be extended to 5,000 words. Systematic reviews, with or without meta-analysis, can include up to 60 references.
Original articles must follow the structure below:
- Title
- Structured abstract
- Keywords
- Introduction
- Methods
- Results
- Discussion
- Acknowledgements (if applicable)
- References
It is recommended that the Methods section include the following information or subsections: i) study design; ii) study population; iii) procedures or interventions; iv) variables; v) data analysis; and vi) ethical aspects.
As part of the editorial evaluation process, all manuscripts must adhere to the appropriate reporting checklist according to the study design, to ensure sufficient methodological detail is provided (http://www.equator-network.org). Observational or quasi-experimental studies (e.g., comparative studies, analyses of large databases, or pre-post intervention evaluations) must follow the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist. Qualitative studies must comply with either the Consolidated Criteria for Reporting Qualitative Research (COREQ) or Standards for Reporting Qualitative Research (SRQR) checklists. Diagnostic accuracy studies must follow the Standards for Reporting Diagnostic Accuracy Studies (STARD checklist); economic evaluations must adhere to the Consolidated Health Economic Evaluation Reporting Standards( CHEERS) guidelines; and experimental animal studies must comply with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. Authors are strongly encouraged to visit http://www.equator-network.org/reporting-guidelines, download the checklist most appropriate to their study design, complete it, and include it as part of the manuscript submission package.
For systematic reviews, with or without meta-analysis, registration is required through PROSPERO (https://www.crd.york.ac.uk/PROSPERO/), the Open Science Framework (https://osf.io/), or another institutional registry. The registration number must be included at the end of the abstract. The reporting of this type of study must follow the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (https://www.equator-network.org/reporting-guidelines/prisma/). Similarly, all clinical trials must be registered in a public trials registry, in accordance with ICMJE recommendations. Registration should occur prior to the enrollment of participants or, at the latest, at the time of first participant inclusion. The registration number must be reported at the end of the abstract. Clinical trial reporting must adhere to the CONSORT (Consolidated Standards of Reporting Trials) guidelines (https://www.consort-spirit.org/).
Brief original articles
Brief original articles are concise manuscripts which, due to the nature of their objectives, design, or findings, can be effectively communicated in a shorter format. This category includes preliminary results from larger research projects or methodological developments that are suitable for summary presentation. The maximum length for a brief original article is 2,000 words, excluding the abstract, acknowledgments (see Authorship Criteria and Contributions section), figure legends, tables, references, and supplementary material. A structured abstract of no more than 150 words is required. Manuscripts may include up to four tables or figures and a maximum of 20 references. Brief original articles may follow the same structure as full original articles, including Introduction, Methods, Results, and Discussion. However, due to their concise nature, a more succinct presentation is expected, while maintaining scientific rigor.
Review articles
Review articles must provide a comprehensive synthesis of the available literature and address topics that fall within the scope of Medical Education & Clinical Practice. The maximum length is 5,000 words, excluding the abstract, acknowledgments (see Authorship Criteria and Contributions section), figure legends, tables, references, and supplementary material. An unstructured abstract of no more than 150 words is required. Manuscripts may include up to seven tables or figures and must contain at least 45 references. Review articles may be structured as follows: an unstructured abstract, keywords, introduction, main content (organized at the author's discretion), a discussion section including conclusions, and references. This category includes narrative reviews, rapid reviews, and scoping reviews.
Special articles
Manuscripts in this section address topics related to public health or medical education, as well as innovative or alternative techniques, methodologies, or approaches relevant to these fields. This category also includes clinical practice guidelines. In some cases, the Editorial Committee of Medical Education & Clinical Practice may invite experts to contribute manuscripts of this nature. The maximum length for a special article is 3,500 words, excluding the abstract, acknowledgments (see Authorship criteria and contributions section), figure legends, tables, references, and supplementary material. An unstructured abstract of no more than 150 words is required. Manuscripts may include up to four tables or figures and a maximum of 40 references. Special articles should be structured as follows: an unstructured abstract, keywords, introduction, main content (including methodological approach, results, and discussion), conclusions, and references.
Study protocol
This type of article presents the protocol of a study that is either in the planning phase or currently underway. Preference is given to protocols for cross-sectional studies with a national or multicenter scope, biobanks, cohort studies, and clinical trials. The maximum length for a study protocol is 3,000 words (excluding the abstract, acknowledgments [see Authorship Criteria and Contributions section], figure legends, tables, references, and supplementary material). An unstructured abstract of no more than 150 words is required. Manuscripts may include up to four tables or figures and a maximum of 30 references. The manuscript must include the following structure: introduction, study hypothesis, study objectives, a detailed description of the proposed methodology, ethical aspects, dissemination plan, and references.
Authors are encouraged to follow the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) checklist (https://www.equator-network.org/reporting-guidelines/spirit-2013-statement-defining-standard-protocol-items-for-clinical-trials/) to ensure a clear and comprehensive presentation of clinical trial protocols.
All submitted protocols must have prior approval from a research ethics committee. For randomized clinical trials, compliance with CONSORT guidelines is required, along with registration in a recognized trial registry, such as ClinicalTrials.gov (https://clinicaltrials.gov/), the WHO International Clinical Trials Registry Platform (ICTRP) (https://www.who.int/clinical-trials-registry-platform), or a national platform officially recognized by the relevant regulatory authority. Observational studies must adhere to the STROBE checklist.
Case report with systematic review
This section is intended for the presentation of clinical cases that address novel aspects of a disease, describe previously undocumented pathological entities, report unusual clinical presentations, highlight new associations in pathological processes, reveal unexpected links between diseases or symptoms, document previously unreported adverse events, or propose novel therapeutic interventions or alternative uses of existing medications.
All case reports must be accompanied by a systematic review of the literature, aimed at contextualizing the case within current scientific knowledge, identifying gaps in the existing evidence, and emphasizing the clinical relevance of the reported finding. The review must follow PRISMA guidelines, including a clearly defined search strategy, inclusion and exclusion criteria, and a synthesis of relevant studies. The review must also be registered in PROSPERO, and the registration number must be included in the abstract.
The maximum length for a case report is 1,500 words, with an unstructured abstract of no more than 150 words, up to five tables or figures, and a maximum of 20 references. The manuscript should follow this structure: unstructured abstract, introduction, case presentation, discussion, acknowledgments, and references. Case reports must comply with the CARE guidelines (https://www.equator-network.org/reporting-guidelines/care/) to ensure clear and precise reporting. Informed consent from the patient or their legal representative must be obtained, as well as authorization from the head of the department or service of the healthcare institution. Patient confidentiality must be strictly maintained (see Informed Consent section).
All images and photographs submitted as part of the case evidence must be of high resolution (above 600 dpi or 300 pixels per inch) and submitted separately in .jpg or .tiff format. The corresponding author must be a medical doctor, and the treating physicians are expected to be included as co-authors.
Letters to the Editor
Scientific letters
These typically consist of descriptive studies based on small, non-probabilistic samples. They may also include case series or reports that highlight clinically relevant findings warranting rapid dissemination. This section also accepts case reports that are not accompanied by a systematic review of the literature. Scientific letters do not require an abstract and must not exceed 1,000 words. They may include one table or figure and up to six references. The structure of a scientific letter should include the following sections: title, main text, and references.
Commentary letters
Commentary letters, non-scientific communications that do not present original research findings, must not exceed 500 words. These letters can serve as critical or clarifying responses to articles published in the latest issue of the journal. They may also offer evidence-based opinions on topics related to medical education, health policy, professional practice, or issues that raise potential ethical concerns in connection with previously published material. Authors cited in commentary letters have the right to submit a reply.
Manuscript format and style
Language
Manuscripts may be written and submitted in either English or Spanish. However, authors are encouraged to use the language in which they can express their ideas with the greatest clarity and precision. Additionally, the text should be written clearly and using inclusive language, considering that the readership may come from a variety of disciplines both within and beyond the health sciences.
Text formatting
Medical Education & Clinical Practice adheres to the International System of Units (SI) in all its articles. Scientific names of species must be written in italics. For manuscripts written in Spanish, a comma should be used as the decimal separator, whereas in English-language texts, a period should be used. Article titles must not contain abbreviations. If abbreviations are used in the body of the text, the full term must be written out at first mention, followed by the abbreviation in parentheses. When reporting measures of association, such as prevalence ratios and their corresponding confidence intervals (CI), two decimal places should be used. For p-values, up to three decimal places are allowed, and for proportions, one decimal place should be used.
Tables
Tables must be placed at the end of the manuscript, after the References section, with each table presented on a separate page. Tables should be numbered consecutively using Arabic numerals and must include a clear title, along with sufficient information to be understood independently of the main text.
To ensure compatibility with the editorial process, tables must be created in editable formats such as Word or Excel. Tables submitted as images (.jpg, .png, or other graphic formats) will not be accepted. All abbreviations and symbols used in the tables must be explained in footnotes, along with any necessary legends.
Figures
Figures may include statistical graphs, flowcharts, diagrams, clinical images, or maps. All figures must be submitted as separate files. Statistical graphs and diagrams should preferably be created in Excel or generated using statistical software. Clinical images, photographs, and maps must be submitted as independent files in .TIFF or .JPG format, with a minimum resolution of 600 dpi or 300 pixels per inch to ensure print quality during layout.
Figures must be numbered consecutively using Arabic numerals and should include a clear title and sufficient information to be understood independently of the main text. Figure titles must appear below each figure.
If a figure includes a patient’s face, their identity must be protected by obscuring the eyes or displaying only what is strictly necessary for case interpretation. In such cases, a signed written consent from the patient or their legal representative authorizing image use must be provided.
If previously published figures are included, the original source must be cited, and written permission from the copyright holder must be submitted.
Supplementary material
Medical Education & Clinical Practice accepts the submission of supplementary material intended to support and enhance the presentation of scientific findings. However, only material directly related to the manuscript’s content will be considered for publication, and its final acceptance is subject to the Editor’s discretion. Approved supplementary material will not be translated and will be published electronically in the format in which it was received. Moreover, supplementary material will not undergo editorial revision and will be published exactly as submitted. All supplementary content must be provided in a single file, while any datasets included as part of the supplementary material may be submitted as an additional file. All supplementary files must be numbered and referenced in the main text. Figures should be labeled consecutively as Figure S1, S2, etc.; tables as Table S1, S2, etc.; and other documents as Supplementary material 1, 2, etc. Supplementary material will be included before the reference list under the subtitle “Supplementary Material”.





